| 培养基名称: | TSC琼脂;TSC培养基、产气荚膜梭菌琼脂;胰蛋白示亚硫酸盐环丝氨酸琼脂基础、胰月示亚硫酸盐环丝氨酸琼脂基础、胰胨亚硫酸盐环丝氨酸琼脂基础、胰胨-亚硫酸盐-环丝氨酸琼脂基础 |
|---|---|
| 英文名称: | TSC Agar;Perfringens Agar;Tryptose Sulfite Cycloserine Agar |
| 培养基类型: | 选择性培养基 |
| 级别: | for microbiology |
| 品牌: | ELITE-MEDIA |
| 产品目录号: | M715-01、M715-02 |
| 产品规格: | 250g、500g |
| 保存条件: | 避光,密封,阴凉干燥处保存。配制好的培养基于2~8度保存,放置时间不得超过7天。 |
| 产品外观: | 淡米色,均一、自由流动的粉末。 |
| 颜色与澄清度: | 浅黄棕色,透明凝胶。 |
| 注意事项: | 避免摄入、呼入、皮肤接触。 |
| 相关产品: |
产气荚膜梭菌肉汤 产气荚膜梭菌琼脂(SFP) 产气荚膜梭菌琼脂(OPSP) |
产品描述:
Perfringens Agar(TSC)产气荚膜梭菌琼脂(TSC)又称TSC琼脂。产气荚膜梭菌琼脂(TSC)是选择性培养基,用于从食品、临床样品及其它材料中分离和增菌产气荚膜梭菌(Clostridium perfringens)。酪蛋白胨亚硫酸盐环丝氨酸琼脂(TSC琼脂)采用D-环丝氨酸(400mg/L)作为筛选剂。焦亚硫酸钠和柠檬酸亚铁铵是亚硫酸还原(H2S生成)指示剂,产气荚膜梭状芽胞杆菌能够还原亚硫酸盐,菌落变黑。卵黄乳液的引入是为了检测该菌的卵磷脂酶活性,同时也促进产气荚膜梭菌的生长,但在计数时不添加卵黄乳液会效果更好。某些产气荚膜梭状芽胞菌株具有卵磷脂酶活性,菌落周围有透明圈产生。然而,不是每株菌都具有产卵磷脂酶能力,因此卵磷脂酶活性阴性的黑色菌落也应该被假定为产气荚膜梭状芽胞杆菌。
用途
产气荚膜梭菌琼脂(TSC)用于从食品、临床样品及其它材料中分离和增菌产气荚膜梭菌(Clostridium perfringens)。在使用TSC琼脂进行产气荚膜梭菌计数时,一般不加卵黄乳液,以获得较小菌落。配方与配制方法
| 成分 | g/L |
| 胰蛋白示 | 15.0 |
| 大豆蛋白胨 | 5.0 |
| 酵母提取物 | 5.0 |
| 柠檬酸亚铁铵 | 1.0 |
| 焦亚硫酸钠 | 1.0 |
| 琼脂 | 15.0 |
| pH 7.6 +/- 0.2 |
补充剂:
D-环丝氨酸 200.0mg
卵黄乳液 50ml
配制方法:
TSC琼脂:称取粉末培养基21g,加450ml去离子重悬,浸泡10min。温和加热煮沸1min,使其完全溶解。121°C高温蒸汽灭菌10min。及时取出,不要过度加热。待培养基冷却至约55°C,加入50ml 50%卵黄乳液,200mg D-环丝氨酸。混匀后倒平板。
接种:接种0.1ml待测样品的梯度浓度水稀释液,涂布均匀。
无卵黄乳液TSC琼脂(上层琼脂):冷却至约50°C,500ml培养基中加200mg D-环丝氨酸,混匀后倒在TSC琼脂平板上面。
使用方法
在使用TSC琼脂进行产气荚膜梭菌计数时,一般不加卵黄乳液,以获得较小菌落。培养温度推荐使用多个温度35-45°C。高温可以促进背景菌落的生长,但是会降低回收率。1.Make up the medium according to the directions and prepare plates containing approximately 20ml of a basal layer of TSC or SFP Agar containing egg yolk.
2.Prepare 0.1ml aliquots of a suitable series of dilutions of the homogenised test sample and spread over the surface of the basal layer using a sterile swab.Overlay with an additional 10ml of egg yolk free TSC or SFP Agar. Cultures which are not overlaid with agar are unlikely to grow as black colonies.
3.Incubate the plates at 35°C for 18-24 hours with an anaerobic Gas Generating Kit in a gas-jar. Alternatively, use AnaeroGen . AnaeroGen does not require the addition of water or a catalyst.
4.Alternatively, pour-plates using approximately 25ml per plate of TSC or SFP Agar containing egg yolk may be prepared using 1ml aliquots of a suitable series of dilutions of the homogenised test sample. Mix the plates well before the agar gels. With this technique, lecithinase activity ofClostridium perfringens colonies is more difficult to see. Clostridium perfringens colonies may be seen as large, black (2-4mm diameter) colonies within the depth of the agar.
Egg yolk free TSC Agar is used with the techniques described above. Clostridium perfringens colonies are black, but, in the absence of egg yolk, no lecithinase activity can be detected.Tests for confirmation are described in a study initiated by the International Commission on Microbiological Specifications for Foods6 involving nitrate reduction, lactose fermentation, gelatin liquefaction and the absence of motility. All black colonies growing on TSC or SFP Agars should be tested.
结果分析:
TSC琼脂支持其它亚硫酸还原型梭菌的生长,因此在TSC琼脂上获得黑色菌落只是假定为产气荚膜梭菌。
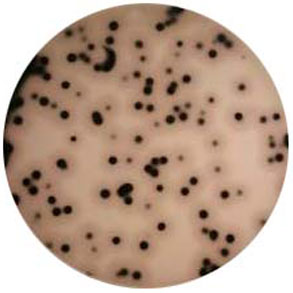
>
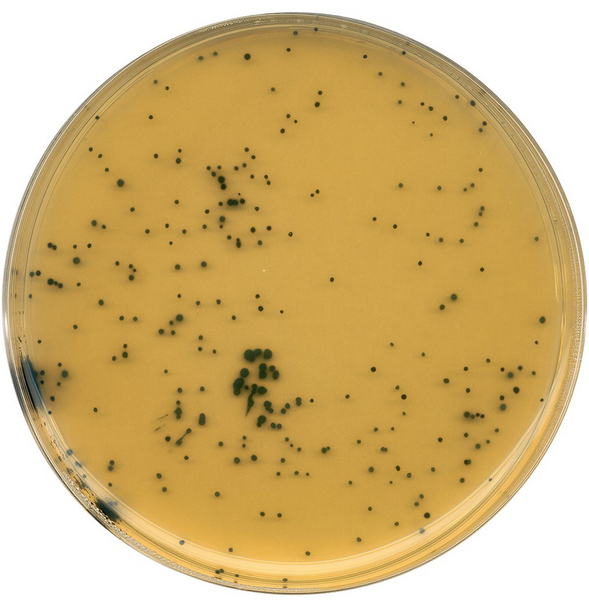
标准菌株在产气荚膜梭菌琼脂(TSC)中的生长情况
标准菌株在44°C下培养18-24小时后,菌落特征如下:
| 标准菌株: | ATCC编号: | 生长情况: | 菌落变黑: | 卵磷脂酶活性: |
|---|---|---|---|---|
| 产气荚膜梭菌 | ATCC13124 | +++ | + | + |
| 破伤风梭状芽胞杆菌 | ATCC19406 | -/++ | - | |
| 诺维氏梭状芽胞杆菌 | ATCC10543 | -/++ | - | |
| 铜绿色假单胞菌 | ATCC27853 | -/+ | - | |
| 蜡样芽胞杆菌 | ATCC11778 | -/+ | - |
产气荚膜梭菌的鉴定——《细菌学鉴定手册》
Bacteriological Analytical Manual Chapter 16 Clostridium perfringens Authors: E. Jeffery Rhodehamel (ret.) and Stanley M. Harmon (ret.) Food poisoning caused by Clostridium perfringens may occur when foods such as meat or poultry are cooked and held without maintaining adequate heating or refrigeration before serving. The presence of small numbers of C. perfringens is not uncommon in raw meats, poultry, dehydrated soups and sauces, raw vegetables, and spices. Because the spores of some strains are resistant to temperatures as high as 100°C for more than l h, their presence in foods may be unavoidable. Furthermore, the oxygen level may be sufficiently reduced during cooking to permit growth of the clostridia. Spores that survive cooking may germinate and grow rapidly in foods that are inadequately refrigerated after cooking. Thus, when clinical and epidemiological evidence suggests thatC. perfringens is the cause of a food poisoning outbreak, the presence of hundreds of thousands or more of these organisms per gram of food substantiates the diagnosis. Illness typically occurs 8-15 h after ingestion of the contaminated food. The symptoms, which include intense abdominal cramps, gas, and diarrhea (nausea and vomiting are rare), have been attributed to a protein enterotoxin produced during sporulation of the organism in the intestine. The enterotoxin can be detected in sporulating cultures, and a method for this purpose is included. A high correlation has been established between the ability of C. perfringens strains to produce enterotoxin and their ability to cause food poisoning. However, it is difficult to obtain consistent sporulation with some strains. C. perfringens cells lose their viability when foods are frozen or held under prolonged refrigeration unless special precautions are taken. Such losses may make it difficult to establish C. perfringens as the specific cause of a food poisoning outbreak. It is recommended that samples which cannot be examined immediately be treated with buffered glycerin-salt solution and stored or shipped frozen to the laboratory as described below. A. Sampling Sample the entire portion of food (whole roast, chicken, gravy, etc.) or take representative samples of 25 g each from different parts of the suspect food because contamination may be unevenly distributed. B. Transporting and storage of samples Transport and examine samples promptly without freezing, if possible, and store at about 10°C until examined. If analysis cannot be started within 8 h or if the sample must be shipped to the laboratory for analysis, treat it with sterile buffered glycerin-salt solution, store immediately at -70 to -90°F, and transport it to the laboratory with dry ice, as described below. Use aseptic technique to prepare sample for storage or shipment. Transfer 25 g portion of sample (sliced beef, turkey, hash, etc.) to sterile 150 ml container, such as plastic Whirl-Pak bag. Add 25 ml buffered glycerin-salt solution, exclude air from bag, and mix the sample well with glycerin solution. Liquid samples such as gravy or beef juice should be mixed well with equal volume of double strength buffered glycerin-salt solution. Store glycerin-treated samples immediately at -70 to -90°F in low temperature freezer or with dry ice so that freezing occurs as quickly as possible. Maintain samples at this temperature until analysis. Thaw samples at room temperature and transfer sample and glycerin-salt solution to sterile blender jar. Add 200 ml peptone dilution fluid to blender jar and proceed with examination. If sample must be shipped to the laboratory, follow procedures above and pack frozen sample in contact with dry ice to maintain temperature as low as possible during shipment. Pack sample in a container such as a paint can or Nalgene bottles which are impervious to CO gas, because absorption of CO2 by the sample could lower the pH and diminish the viability of C. perfringens. Store sample at -70 to -90°F on receipt and keep at this temperature until examined, preferably within a few days. Cultural Methods for Enumeration and Identification of Clostridium perfringens in Foods A. Equipment and materials 1. Pipets, 1.0 ml with 0.1 ml graduations, and 10.0 ml with 1.0 ml graduations 2. Colony counter 3. High speed blender, Waring or equivalent, and 1 L glass or metal blender jars with covers; 1 jar required for each sample 4. Anaerobic jars, BBL GasPak, or Oxoid anaerobic jars equipped with GasPak H2 + CO2 generator envelopes and catalyst 5. Incubator, 35°C 6. Petri dishes, sterile 15 × 100 mm 7. Platinum loop, 3 mm id 8. Water bath, 46 ± 0.5°C 9. Reversed passive latex agglutination (RPLA) test kit for C. perfringens enterotoxin (Oxoid USA, Columbia, MD) B. Media and reagents 1. Tryptose-sulfite-cycloserine (TSC) agar 2. Egg yolk emulsion, 50% 3. Chopped liver broth or cooked meat medium (modified) (M43) (chopped liver is preferred) 4. Thioglycollate medium (fluid) 5. Iron milk medium (modified) 6. Lactose-gelatin medium (for C. perfringens) 7. Sporulation broth (for C. perfringens) 8. Motility-nitrate medium, buffered (for C. perfringens) 9. Spray's fermentation medium (for C. perfringens) 10. AE sporulation medium, modified 11. Duncan-Strong sporulation medium, modified 12. Peptone diluent 13. Nitrite detection reagents 14. Glycerin-salt solution (buffered) 15. Gram stain reagents 16. Fermentation test papers. Saturate 15 cm Whatman No. 31 filter paper disks with 0.2% aqueous bromthymol blue solution adjusted to pH 8-8.5 with ammonium hydroxide. Air-dry the disks and store for later use. 17. Bromthymol blue, 0.04% aqueous solution C. Cultural and isolation procedures Prepare Gram stain of sample and examine for large Gram-positive rods. Plate count of viable C. perfringens. Using aseptic technique, place 25 g food sample in sterile blender jar. Add 225 ml peptone dilution fluid (1:10 dilution). Homogenize 1-2 min at low speed. Obtain uniform homogenate with as little aeration as possible. Using 1:10 dilution prepared above, make serial dilutions from 10-1 to 10-6 by transferring 10-90 ml peptone dilution fluid blanks. Mix each dilution thoroughly by gently shaking before each transfer. Pour 6-7 ml TSC agar without egg yolk into each of ten 100 × 15 mm petri dishes and spread evenly on bottom by rapidly rotating dish. When agar has solidified, label plates, and aseptically transfer 1 ml of each dilution of homogenate to the center of duplicate agar plates. Pour additional 15 ml TSC agar without egg yolk into dish and mix with inoculum by gently rotating dish. An alternative plating method preferred for foods containing other types of sulfite-reducing organisms is to spread 0.1 ml of each dilution with sterile glass rod spreader over previously poured plates of TSC agar containing egg yolk emulsion. After inoculum has been absorbed (about 5 min), overlay plates with 10 ml TSC agar without egg yolk emulsion. When agar has solidified, place plates in upright position in anaerobic jar. Establish anaerobic conditions and place jar in 35°C incubator for 20-24 h. (TSC agar containing egg yolk is incubated 24 h.) After incubation, remove plates from anaerobic jar and select those containing 20-200 black colonies for counting. C. perfringens colonies in egg yolk medium are black with a 2-4 mm opaque white zone surrounding the colony as a result of lecithinase activity. Using Quebec colony counter with white tissue paper over counting area, count black colonies and calculate number of clostridia cells/g food. Save plates for identification tests (see D, below). Prepare chopped liver broth (or cooked meat medium) for inoculation by heating 10 min in boiling water or flowing steam and cooling rapidly without agitation. Inoculate 3 or 4 broth tubes with 2 ml of 1:10 homogenate as back-up for preceding plating procedure. Incubate these tubes 24-48 h at 35°C in standard incubator. Disregard if plate counts for viable C. perfringens are positive. D. Presumptive confirmation test Select 10 typical C. perfringens colonies from TSC or TSC-egg yolk agar plates and inoculate each into a tube of freshly deaerated and cooled fluid thioglycollate broth. Incubate in standard incubator 18-24 h at 35°C. Examine each culture by Gram stain and check for purity. C. perfringens is a short, thick, Gram-positive bacillus. If there is evidence of contamination, streak contaminated culture(s) on TSC agar containing egg yolk and incubate in anaerobic jar 24 h at 35°C. Surface colonies of C. perfringens are yellowish gray with 2-4 mm opaque zones caused by lecithinase activity. This procedure is also used for isolating C. perfringens from chopped liver broth whenever the organism is not detected by direct plating on TSC agar. Iron-milk presumptive test. Inoculate modified iron-milk medium with 1 ml of actively growing fluid thioglycollate culture and incubate medium at 46°C in a water bath. After 2 h, check hourly for "stormy fermentation." This reaction is characterized by rapid coagulation of milk followed by fracturing of curd into spongy mass which usually rises above medium surface. Remove positive tubes to prevent spilling over into water bath. For this reason, do not use short tubes for the test. Cultures that fail to exhibit "stormy fermentation" within 5 h are unlikely to be C. perfringens. An occasional strain may require 6 h or more, but this is a questionable result that should be confirmed by further testing. Some strains of C. baratii react in this manner, but this species can be differentiated by its inability to liquefy gelatin in lactose-gelatin medium. The rapidity with which the "stormy fermentation" occurs depends on the strain and the initial population. Therefore, only actively growing cultures are appropriate for this test. The presumptive test in iron-milk medium may be sufficient for some purposes. However, the completed test must always be performed with isolates associated with food poisoning outbreaks. The following tests must be included for the completed test. E. Completed confirmation test Stab-inoculate motility-nitrate (buffered) and lactose-gelatin media with 2 mm loopfuls of pure fluid thioglycollate medium culture or portion of isolated colony from TSC agar plate. Stab lactose-gelatin repeatedly to ensure adequate inoculation, and then rinse loop in beaker of warm water before flaming to avoid splattering. Incubate inoculated media 24 h at 35°C. Examine lactose-gelatin medium cultures for gas production and color change from red to yellow, which indicates acid production. Chill tubes 1 h at 5°C and examine for gelatin liquefaction. If medium gels, incubate an additional 24 h at 35°C and examine for gelatin liquefaction. Inoculate sporulation broth with 1 ml fluid thioglycollate medium culture and incubate 24 h at 35°C. Prepare Gram stain of sporulation broth and examine microscopically for spores. Store sporulated cultures At 4° if further testing of isolates is desired. C. perfringens is nonmotile. Examine tubes of motility-nitrate medium for type of growth along stab line. Nonmotile organisms produce growth only in and along stab. Motile organisms usually produce diffuse growth out into the medium, away from the stab. C. perfringens reduces nitrates to nitrites. To test for nitrate reduction, add 0.5 ml reagent A and 0.2 ml reagent B (R48) to culture in buffered motility-nitrate medium. Violet color which develops within 5 min indicates presence of nitrites. If no color develops, add a few grains of powdered zinc metal and let stand a few minutes. A negative test (no violet color) after zinc dust is added indicates that nitrates were completely reduced. A positive test after addition of zinc dust indicates that the organism is incapable of reducing nitrates. Tabulate results. C. perfringens is provisionally identified as a nonmotile, Gram-positive bacillus which produces black colonies in TSC agar, reduces nitrates to nitrites, produces acid and gas from lactose, and liquefies gelatin within 48 h. Some strains of C. perfringens exhibit poor sporulation in sporulation medium or weak lecithinase reactions on TSC agar containing egg yolk. Organisms suspected to be C. perfringenswhich do not meet the stated criteria require additional testing for confirmation. Subculture isolates which do not meet all criteria for C. perfringens into fluid thioglycollate medium. Incubate 24 h at 35°C, prepare Gram stain, and examine for purity and typical cell morphology. Inoculate 0.1 ml pure fluid thioglycollate culture into 1 tube of freshly deaerated Spray's fermentation medium containing 1% salicin, 1 tube containing 1% raffinose, and 1 tube of medium without carbohydrate. Incubate media 24 h at 35°C and examine medium containing salicin for acid and gas. Test for acid by transferring a 2 mm loopful of culture to bromthymol blue test paper. Use only a platinum loop. No color change or development of a slight green color indicates that acid was produced. Alternatively, transfer 1.0 ml of culture to test tube or spot plate and add 1 or 2 drops of 0.04% bromthymol blue. A light green or yellow color indicates that acid was produced. Incubate media for another 48 h and test for acid production. Salicin is rapidly fermented with production of acid and gas by culturally similar species but usually is not fermented by C. perfringens. Acid is usually produced from raffinose within 3 days by C. perfringens but is not produced by culturally similar species. A slight change in pH can occur in the medium without fermentation of carbohydrates. Some species of Clostridium occasionally isolated from foods have characteristics which differentiate them from C. perfringens. C. paraperfringens and C. baratii – slender cells frequently in filamentous chains with large spherical bodies in cooked meat or other media containing carbohydrate; nitrite weak or absent after 18 h; very weak lecithinase production; gelatin never liquefied. C. absonum or C. sardiniensis – young cultures may exhibit weak motility; gelatin slowly liquefied; strong lecithinase production; nitrite production weak or absent after 18 h. C. celatum – similar to C. paraperfringens, except that cells form large mass in bottom of tube; usually grows very slowly; all reported isolates of C. celatum are from feces. C. celatum differs from C.paraperfringens by the absence of lecithinase activity and by the production of acid from starch. Calculate number of C. perfringens cells in sample on the basis of percent of colonies tested that are confirmed as C. perfringens. Example: If average plate count of 10-4 dilution was 85, and 8 of 10 colonies tested were confirmed as C. perfringens, the number of C. perfringens cells/g food is 85 × (8/10) × 10,000 = 680,000. NOTE: The dilution factor with plates containing egg yolk is tenfold higher than that of the sample dilution because only 0.1 ml was plated. F. Culturing procedures for sporulation and enterotoxin production If isolates are to be tested immediately for sporulation and enterotoxin production, subculture in fluid thioglycollate broth as described above. Cultures to be stored or shipped to another laboratory for testing should be subcultured in Difco cooked meat medium and incubated for 24 h at 35°C, followed by an additional 24 h at room temperature. Store cooked meat culture at 4°. To subculture for sporulation and enterotoxin production, mix cooked meat culture with Vortex mixer and transfer 0.5 ml of the mixture to each of two tubes containing 10 ml of freshly steamed fluid thioglycollate medium. Heat one tube in a beaker of water or in a water bath at 75°C for 10 min, and incubate at 35°C for 18 h. Incubate the second tube at 35°C for 4 h, and use this culture to inoculate modified AE sporulation medium. For best results use 0.75 ml of 4 h thioglycollate culture to inoculate 15 ml of modified AE or modified Duncan-Strong sporulation media. Incubate inoculated spore broth at 35°C in anaerobic jar or incubator for 18-24 h. Check resulting culture for spores by using a phase-contrast microscope or by examining stained smears. Fewer than 5 spores per microscopic field is not considered good sporulation.


